Introduction: TP53 mutations in patients with myeloproliferative neoplasms (MPN) are associated with poor prognosis, including progression to blast phase MPN. However, low variant allele fraction (VAF) TP53 mutations have been reported to remain stable over years in chronic phase MPN. A major unmet clinical need in MPN is the ability to discriminate patients with TP53-mutant MPN who are at high-risk of secondary AML (sAML) and warrant immediate intervention from those who are at lower risk of sAML in whom active surveillance can be employed. Therefore, we sought to identify parameters associated with leukemic transformation and overall survival in the context of MPN with genetic aberrations in TP53.
Materials and Methods: We retrospectively analyzed a cohort of 947 MPN patients from the Dana-Farber Cancer Institute Hematologic Malignancies Data Repository (HMDR) with at least one clinical next-generation sequencing (NGS) panel performed. Patient characteristics such as age at MPN diagnosis, gender, MPN subtype and driver mutations were recorded. Furthermore, information about the course of disease was extracted including occurrence of sAML and overall survival (Figure 1). We also analyzed type and number of additional mutations as well as cytogenetics. With respect to TP53-specific parameters, we evaluated the number of TP53 mutations, TP53 VAF, loss of heterozygosity (LOH) at the TP53 locus, phenotypic annotations of TP53 (i.e. PHANTM score) and 17p deletion. We defined “multi-hit” TP53 as the presence of two or more TP53 mutations, TP53 VAF higher than 50%, TP53 mutation plus 17p deletionor TP53 mutation and documented LOH.
Results: A total of 947 patients were analyzed, of which 40 harbored at least one detectable TP53 mutation. A total of 13 patients were found to have a multi-hit TP53 mutations defined by > 50% VAF in 6 patients, two or more TP53 mutations in 5 patients and TP53 mutation + 17p deletion in 5 patients. The MPN diagnosis at time of TP53 mutation detection was post ET/PV myelofibrosis (secondary MF) (n=23, 58%), primary myelofibrosis (MF) (n=7, 18%), pre-fibrotic MF (n=2, 5%), essential thrombocythemia (ET) (n=6, 15%) and polycythemia vera (PV) (n=2, 5%). Two patients with ET and one patient with PV did not have a concurrent in-house bone marrow biopsy performed at the time the TP53 mutation was detected. Two patients with ET developed sAML within 12 months of TP53 mutation detection, without prior mention of fibrosis. Age at first MPN diagnosis was not significantly different between patients with or without TP53 mutation. The average time from initial MPN diagnosis to detection of the first TP53 mutation was 9 years (range: 0-33 years). The most common MPN driver mutation among patients with TP53 mutations was JAK2 (75%), followed by CALR (13%)and MPL (5%). Out of all TP53-mutated patients, 8% showed a triple negative status. The most frequent additional mutations among patients with TP53 mutations were TET2 (25%), U2AF1 (15%), ASXL1 (13%), and DNMT3A (10%). There was no significant difference between single-hit and multi-hit TP53 status regarding MPN subtype, driver mutations and co-mutations (Table 1). Seven patients (single-hit: 15%, multi-hit: 23%) with a TP53 mutation developed sAML during the course of their disease, compared with only 3% of all patients without a TP53 mutation and 50% (single-hit: 41%, multi-hit: 69%) were deceased at the time of the last follow-up compared to 18% of all patients without a TP53 mutation.
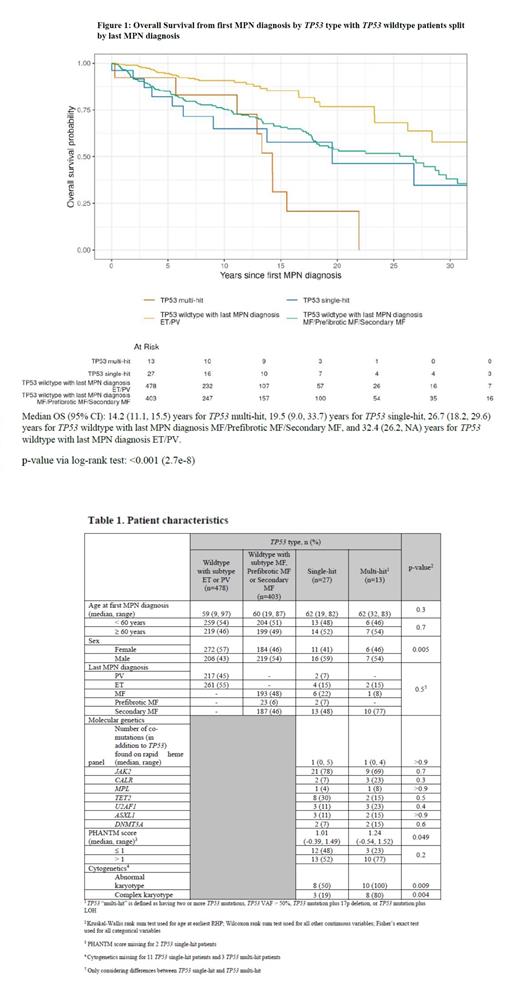
We focused on overall survival from the initial MPN diagnosis and considered whether patients developed bone marrow fibrosis during their disease course (Figure 1). Survival did not differ significantly between single-hit TP53 and patients with multi-hit TP53 (p=0.2), but survival did differ significantly between multi-hit TP53 patients and TP53 wildtype patients with MF/prefibrotic MF/Secondary MF (p=0.02) as well as compared to all MPN patients without a TP53 mutation (p<0.001). Survival was not significantly different between single-hit TP53 and TP53 wildtype MF/prefibrotic MF/Secondary MF patients (p=0.4).
Conclusions: In a large cohort of 947 molecularly characterized MPN patients, 4% of the cohort developed a TP53 mutation during their course of disease. 18% of all TP53-mutant patients developed sAML with an adverse effect on overall survival for patients with multi-hit but not single-hit TP53 mutations.
Disclosures
Luskin:AbbVie: Research Funding; Jazz: Honoraria; Pfizer: Honoraria; Novartis: Honoraria, Research Funding. DeAngelo:Pfizer: Honoraria; Blueprint: Honoraria; Kite: Honoraria; Jazz: Honoraria; Gilead: Honoraria; Amgen: Honoraria; Servier: Honoraria; Novartis: Honoraria; AbbVie: Research Funding; Novartis: Research Funding; Blueprint: Research Funding; GlycoMimetics: Research Funding; Incyte: Honoraria; Autolus: Honoraria; Takeda: Honoraria. Lindsley:Jazz Pharmaceuticals: Consultancy; Verve Therapuetics: Consultancy; Vertex Pharmaceuticals: Consultancy; Sarepta Therapuetics: Consultancy; Takeda Pharmaceuticals: Consultancy; Bluebird bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Qiagen: Consultancy. Weeks:Abbvie: Consultancy. Aryee:SeQure Dx: Consultancy, Current equity holder in private company. Stahl:Boston Consulting: Consultancy; Rigel: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: GME activity ; Sierra Oncology: Membership on an entity's Board of Directors or advisory committees; Haymarket Media: Other: GME activity ; Clinical care options: Other: GME activity ; Dedham group: Consultancy; Curis Oncology: Other: GME activity ; GSK: Membership on an entity's Board of Directors or advisory committees; Kymera: Membership on an entity's Board of Directors or advisory committees. Mullally:AOP Health: Speakers Bureau; Aclaris, Cellarity, Morphic, Biomarin: Consultancy; Relay, Morphic: Research Funding; PharmaEssentia, Incyte: Other: Steering Committee ; Constellation, Protagonist: Other: Advisory Board .